| |
Prepared in collaboration with Timothy M Tan
Outline of Sections
|
Section 1 |
|
Section 2 |
|
Section 3 |
|
3.1 |
|
3.2 |
|
Section 4 |
|
Section 5 |
|
5.1 |
|
5.2 |
|
Section 6 |
|
6.1 |
|
6.2 |
|
Section 7 |
|
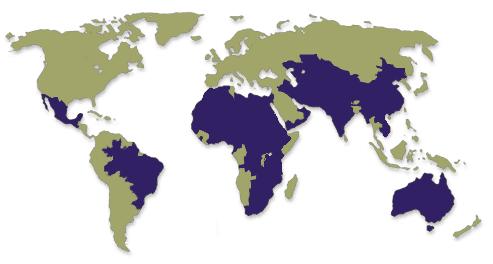
Trachoma, an infection of the eye caused by Chlamydia trachomatis, ranks worldwide as the most common preventable cause of blindness and the second most common cause of blindness after cataract. It has been estimated to cause 15% of the world's blindness.1,20 The disease is endemic in 48 countries in Latin America, Africa, the Middle East, Asia, and Australasia [see Fig. 1], and is most prevalent in poor, rural communities with lower standards of hygiene and sanitation.2 The WHO currently estimates that 6 million people are blind due to trachoma, and that an additional 146 million people have active forms of the disease. Furthermore, morbidity arising from trachoma is estimated to cost US $2.9 billion per year in lost workforce productivity.1
 |
|
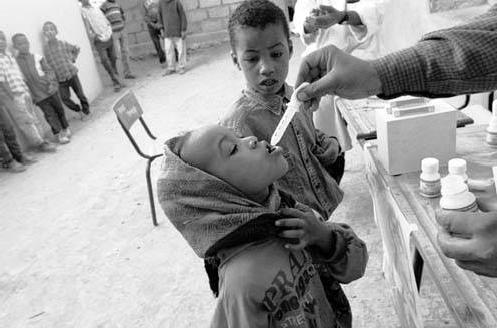
In 1997, the WHO organized the Alliance for Global Elimination of Trachoma by 2020 (GET 2020) and recommended the 'SAFE' strategy as a basic framework for dealing with trachoma. This strategy involves the use of Surgery to treat advanced stages of disease, Antibiotic treatment with azithromycin, and the promotion of Facial cleanliness and Environmental change. Early evaluations of ten national trachoma elimination campaigns already show promising results for the first two interventions, namely eyelid surgery and administration of antibiotic treatment.1 Improving facial hygiene and encouraging environmental change, however, has proven more difficult to implement and assess, yet they are equally important components of the SAFE strategy and vital to the success of any campaign to eliminate blinding trachoma.
 |
|
Recorded knowledge of trachoma dates back as far as 1900 bc , with descriptions appearing in the Egyptian Ebers papyrus.23 Ancient Greek physicians were also familiar with the disease, and Galen described the rough inner lining of the upper eyelid that is symptomatic of repeated infections. Ibn 'Isa, an Arab physician during the 11th century, correctly observed that chronic trachoma infection results in trichiasis, which is a painful condition characterized by infolding of the upper eyelid such that the eyelashes scratch the cornea.1 European experiences with trachoma were first noted during the French occupation of Egypt in the late 18th century. During the 19th and early 20th centuries, trachoma emerged as a well-documented public health problem in England, parts of Europe, and the United States, with several nations forming eye hospitals whose specific aim was the treatment and quarantine of the disease.1,2 Screening for trachoma was even performed on Ellis Island, and tens of thousands of potential immigrants to the United States were sent back to Europe due to trachoma infection.1,2,23 With improvements in the standard of living in Europe and the United States, however, trachoma was effectively eradicated in the industrialized West by the early 1950s, though it remains prevalent in other, less-developed parts of the world.
Cytoplasmic inclusions from infected conjunctival epithelial cells were first visualized in 1907 and recognized as the causative agent in trachoma infection.2,23 Each of these cytoplasmic inclusions contained numerous individual organisms, but fifty years passed before these organisms were successfully isolated and cultured in hens' eggs by Tang et al in 1957. The organism, now known to be the bacterium Chlamydia trachomatis , was then used to inoculate a blind volunteer at London's Institute of Ophthalmology, and the subsequent development of symptoms of trachoma in the volunteer confirmed that C. trachomatis was indeed the infective organism responsible for the disease. 23
Infection with Chlamydia trachomatis is known to result in a number of diseases in humans. The specific ocular serotypes of C trachomatis responsible for the conjunctival infections of trachoma are serotypes A, B, Ba, and C.2 Human infection by serotypes D-K typically occurs in the genital tract and related areas, where it presents as a sexually transmitted disease (STD) such as pelvic inflammatory disease and tubal factor infertility, but can also be implicated in conjunctivitis and neonatal pneumonia.13,21 The rare L1, L2, and L3 serotypes are responsible for lymphogranuloma venerum.2,13 Transmission of trachoma infection from human to human can be by contact with surfaces containing the ocular serotypes of C. trachomatis in the form of spore-like elementary bodies,2 such as through shared towels and bedclothes, or hand-to-eye contact.3 In addition, C. trachomatis transmission can be mediated by flies, including two common muscid species.4
3.1 |
Chlamydia trachomatis and trachoma |
|
|
C. trachomatis is a gram-negative, coccoid bacterium that typically infects columnar epithelial cells.13,21 The bacterium is an obligate intracellular parasite that is dependent on its host cell for replication and as an ATP energy source.2,22 It exhibits a characteristic two-stage developmental lifecycle consisting of spore-like, infectious 'elementary bodies' and replicating, intracellular 'reticulate bodies'.2,21 During the first stage, the extracellular elementary body, which is metabolically inactive, comes into contact with a host eukaryote which actively internalizes the elementary body by phagocytosis.2,22 Following inclusion into a membrane-bound phagosome, the elementary body begins to differentiate into a metabolically active reticulate body, thus entering the second lifecycle stage.21,22 The reticulate body almost immediately alters its intracellular niche by preventing lysosomal degradation of the phagosome. Reticulate bodies then reproduce within the phagosome via several cycles of binary fission. At the end of this stage, the reticulate bodies differentiate back into the elementary body form, and host cell lysis liberates the new elementary bodies which can continue the infectious cycle.21 In vitro , the C. trachomatis cycle of internalization, replication, and release requires 48-72 hours.2
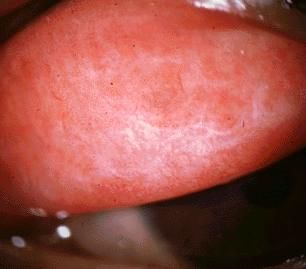
Infection with the ocular serotypes of C. trachomatis results in a mild inflammation of the subtarsal conjunctiva lining the inner eyelid surface. Single episodes of infection usually resolve without any permanent damage. With repeated episodes of infection by C. trachomatis, however, a chronic condition of conjunctivitis results, leading to the formation of sub-epithelial follicles that are visible upon inspection of the inner lining of the upper eyelid [see Fig. 3]. Eventually, chronic reinfection and conjunctival inflammation results in scarring of the subtarsal conjunctiva, with deterioration of the epithelium and depletion of mucus-secreting goblet cells. Such scarring is also characterized by replacement of loose type I and III collagen with tougher, more compact type IV and V collagen .2 This is of particular clinical importance in the upper eyelid, where the scarred inner lining deforms the lid margin causing the eyelid to fold inwards. Inversion of the upper eyelid brings the eyelashes in contact with the cornea of the eye, thereby scratching the surface of the eye with each blink or movement of the eyelid. This painful condition is called trichiasis, and with time the chronic corneal scratching results in the development of opaque corneas and subsequent blindness [see Fig. 4].2
 |
|
| Figure 3. |
Photograph courtesy of C.N. Chua; http://www.mrcophth.com |
|
 Figure 4. Figure 4. |
Photograph courtesy of C.N. Chua;
http://www.mrcophth.com |
|
3.2 |
Transmission of Chlamydia trachomatis |
|
|
A number of observations and research findings indicate that the main source of C. trachomatis infection arises from elementary bodies of the bacteria shed by individuals with active trachoma. Among the evidence for this conclusion is the finding that secretions from infected eyes test positive for C. trachomatis with a high degree of consistency.3 C. trachomatis has also been isolated from nasopharyngeal swabs and nasal exudates.2 Infected children are important reservoirs of the disease in endemic areas and regions with a high prevalence of trachoma, as the load of infection is disproportionately high among children younger than 10 years old.5 Also, Burton, Holland, Faal et al used a PCR-based assay to test for infection loads of C. trachomatis in conjunctival swabs, and their results suggest that in regions of lower trachoma prevalence, infected individuals without clinical signs of active disease still represent a significant reservoir of C. trachomatis.7 Moreover, individuals noted for having nasal and ocular secretions were found to be several times more likely to harbor high infection loads.
The presence in ocular and nasal secretions of significant quantities of C. trachomatis elementary bodies, as well as the substantial prevalence of infection especially among children, indicate that the primary means of trachoma transmission is via contact with infected secretions, fomites, or vectors. This can be summarized by the following four possible routes of infection11:
- Direct contact - eye-to-eye transmission with fingers, such as by touching the eyes or ocular and nasal secretions of infected individuals, and then touching one's own eyes
- Indirect contact - transmission via shared towels, handkerchiefs, bedclothes, etc.
- Eye-seeking flies (discussed in the next section)
- Coughing or sneezing
Due to this variety in possible routes of infection, as well as the likelihood that all of these routes play a significant role in the spread of infection, trachoma control programs must be sufficiently broad-based and multi-faceted to address each possibility. Furthermore, in individual countries or regions, the relative importance of certain routes of infection might be greater than others. Thus, attempts to control trachoma must be sensitive to the subtle differences in the form of local trachoma endemicity, as one standardized, globally-uniform plan for trachoma control would likely be inadequate. 11
A large body of research has implicated several synanthropic muscid flys as putative vectors of ocular C. trachomatis infection. The most important of these species is most likely Musca sorbens, commonly known as the bazaar fly [see Fig. 5]. Musca domestica, the common housefly found throughout the world, may also have a role in trachoma transmission, and M. vetustissima, the bush fly, is significant only in Australia.
 |
| Figure 5. |
Photograph by R.F.L. Mau, College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa |
|
In a series of laboratory tests conducted by Forsey and Darougar, M. domestica was shown to be capable of not only harboring viable C. trachomatis for up to 6 hours in its alimentary tract, but also of transmitting ocular chlamydial infections between guinea pigs.8 M sorbens has also been closely correlated with trachoma through observational studies of the presence of M. sorbens on the faces of children with active disease.9 In fact, M. sorbens is involved in the bulk of fly-eye contacts in several trachoma-endemic regions.18 Perhaps the most compelling evidence for the role of muscid flies as vectors of C. trachomatis comes from a study by Emerson et al , in which insecticide intervention with deltamethrin led to a 75% fall in the muscid population (both M. sorbens and M. domestica ) and a 75% fall in the number of new cases of trachoma.10 And in Australia, the bush fly M. vetustissima has been proposed as a vector of trachoma, both by demonstration of viable C. trachomatis in the intestines 11 to 14 hours after ingestion and by correlation of M. vetustissima seasonality with trachoma incidence.24 All three of these fly species exhibit eye-seeking behavior and are known to feed on eye secretions and nasal exudates,4,8 which as discussed earlier consistently contain the infectious C. trachomatis elementary bodies.
 |
|
In addition to the biological factors that allow these three muscid fly species to harbor and transmit viable elementary bodies of C. trachomatis, various ecological factors contribute to their effectiveness as vectors for trachoma. Of particular interest is the breeding environment favored by the flies, which may explain their synanthropic behavior and the presence of large populations nearby human settlements in trachoma-endemic regions. Studies of the relative significance of various types of feces for M. sorbens breeding indicate that human, calf, cow, dog, and goat feces all represent viable larval growth media.18 Human feces, however, are a particularly good breeding medium for M. sorbens , with larger and more numerous flies consistently emerging from human feces as compared to other animal excrement. Further research has also shown that M. sorbens exhibit a preference for fresh, isolated human feces on the ground, and will not breed in the liquefied excrement found in pit latrines.11,12 As a result, M. sorbens is favorably adapted to cohabitation with humans, especially in parts of the world lacking sewage systems or pit latrines and in which people continue to defecate in open areas.
The ability for M. sorbens to breed, albeit less efficiently, in cattle, dog, and goat feces significant in regions where people keep animals for livestock or as pets. By living alongside or nearby humans, these animals provide favorable breeding conditions for M. sorbens through their feces, especially in circumstances where animal wastes are not actively removed from the open.
5 |
The human perspective |
5.1 |
Risk factors |
|
|
The current distribution of trachoma-endemic regions correlates well with poverty, with high prevalence of trachoma being tightly associated with the poorest parts of the world. Additional environmental and social variables that have been shown to be risk factors for trachoma include heat and aridity, considerable distance from a source of water source and scarcity of water,11,12 rural residence, lack of latrines,13 crowded living conditions, and keeping cattle or other animals close to the home.12 Emerson et al suggest, however, that such correlations might simply be proxies for poverty, and that trachoma is not just a disease of hot and arid locations.11
 |
Figure 8. Photograph by Elizabeth Gilbert. |
| |
|
There is nevertheless some causal explanation for the apparent correlation between trachoma prevalence and heat, dryness, and living distance from a water source. This explanation arises from the general standard of hygiene, and especially personal facial hygiene, and its relationship to the availability of water. As Schémann et al found through a national disease prevalence survey in Mali , families with easier access to a water source tended to use more water for bathing and other hygienic purposes such as laundry.12 Standards of personal hygiene are in turn correlated with prevalence of trachoma, as clean faces are less likely to harbor the bacteria. Schémann reports that trachoma was more prevalent if less than ten liters of water was used to bathe a child,12 and that dirty faces are strongly associated with active disease.14 In addition, West et al further observed in Central Tanzania that nasal discharges are particularly significant in assessing facial dirtiness, with the presence of nasal discharge alone resulting in an odds ratio of 1.7 among children.15 Furthermore, a community-based randomized trial of a face-washing intervention program among several villages in Tanzania showed that after one year, people living in intervention villages had lower odds of presenting with severe trachoma.16 It is also interesting to note that with regards to water, quantity and availability are critical, but the quality of the water supply has little effect on trachoma prevalence.12
Crowding within rooms has been observed in many trachoma-endemic regions to be a risk factor for the disease.7,12 Moreover, active trachoma and C. trachomatis infection tend to present as clusters of disease focused at the shared-room, home or compound, and village levels.11 In study villages in The Gambia, Burton et al found that individuals living in the same compound as a person with active trachoma had an odds ratio of 5.86 for having a high chlamydial infection load, and that the odds ratio was 2.97 for persons sharing a bedroom with at least four other individuals.7 These correlations most likely account for the role of prolonged social and physical contact in the transmission of C. trachomatis by infected fingers, bedclothes or towels, and flies.
The association between trachoma prevalence and the lack of latrines and possession of cattle or other animals close to the home, as mentioned previously, is probably due to the favorable muscid breeding medium found in human and animal feces. This is especially true of human feces, as M. sorbens has been observed to prefer human feces over other types.11 Covered pit latrines thus confer a protective effect against trachoma prevalence by removing human feces from the environment; this has been demonstrated in numerous study areas including The Gambia,7 Mali,12 Yemen,13 Egypt, Malawi, Australia, and Ethiopia.11 Evidence for the significance of animal feces in contributing to muscid breeding and hence trachoma transmission, however, is less concrete. Some studies in The Gambia,7 Ethiopia,17 and Tanzania26 showing increased risk of trachoma with cattle proximity, while another study in Mali12 showed a protective effect conferred by cattle ownership. This protective effect may simply be due to a correlation between cattle ownership and wealth, which is in turn associated with better outcomes concerning trachoma. Nevertheless, various animal feces including the feces of dogs, goats, and cattle are possible larval growth media for M. sorbens and other muscid flies,18 and their involvement should not be ignored.
 |
|
5.2 |
Distribution of trachoma within populations |
|
|
Numerous investigations have shown that children are the main reservoir of C. trachomatis . In a study village in The Gambia, where the highest prevalence of active trachoma was seen in children under five years old, the prevalence diminished with increasing age over five years.7 Furthermore, by using PCR, the prevalence of high-load infections with C. trachomatis followed a similar trend; for low infection loads there was no observed age bias. Similar results were obtained in separate studies involving trachoma-endemic regions of Mali,12 Yemen,13 and Tanzania,5 with the age of peak trachoma prevalence ranging between three and six years. One possible explanation for this trend is that personal hygiene practices tend to develop with age as children become more aware of their hygiene and increasingly capable of caring for themselves.13 Another factor is that episodes of infection and active trachoma generally last for a longer duration in children as compared to adults.25
 |
|
While this correlation between age and trachoma prevalence is generally quite accurate in describing the majority of trachoma-endemic regions of the world, the strength of the correlation appears to decline with lower overall prevalence.5 Nevertheless, even for low-prevalence areas, children below the age of ten years will most likely still serve as the main reservoir of C. trachomatis elementary bodies, and Solomon et al suggest that these individuals should be the principle target for antibiotic campaigns. Moreover, children below the age of one year have been shown to be capable of harboring high loads of C. trachomatis infection, which is a finding that challenges the guidelines in certain nations that restrict the use of azithromycin antibiotic treatment to persons older than one year of age.5
 |
|
In addition to an age bias for higher trachoma prevalence among children, disease prevalence is also differs according to gender. In this regard, there does not appear to be a common trend that describes the burden of trachoma cross-nationally. In some countries, such as Yemen13 and Mali,12 a higher prevalence of active trachoma is found in men (not statistically significant for the Mali study), whereas in other regions including Ethiopia17 and the Kongwa district of Tanzania,6 trachoma prevalence is greater for women, especially above the age of fifteen years. Other studies have shown no gender bias for disease prevalence for study populations in The Gambia and the Rombo district of Tanzania.5
The apparent incongruity of gender biases for trachoma prevalence is very likely the result of complex ecological, social, and cultural interactions that shape unique environments for the pathology and transmission of trachoma. When considering the higher prevalence among women in certain regions, for example, researchers have suggested that increased contact between children and care-taking women may explain the higher infection rates among women.6,12,17 This would also explain the observation in Ethiopia that trachoma prevalence is higher in women only above fifteen years of age,17 and also the finding in Tanzania that the odds ratio for having clinically active trachoma is highest among mothers who care for children (OR=2.43), elevated among non-caretaking mothers (OR=1.63), and least among women without children.6 The social norms shaping this bias, however, might not apply in other parts of the world, in which case different gender distributions of disease prevalence might be expected.
 |
|
6 |
Medical ecology of trachoma |
|
|
In 1997, the WHO launched a campaign for the global elimination of trachoma by 2020 and adopted the 'SAFE' strategy for achieving this goal. The momentum behind this campaign arose from several advances in control methods for trachoma, including the recognition that a single oral dose of azithromycin could effectively cure an infectious episode of trachoma. This represented a vast improvement over the previous standard treatment for trachoma, which involved 6 weeks of local application of tetracycline ointment and was difficult both in terms of compliance and ease of administration.2 To add to the momentum, Pfizer Inc pledged in 1998 to donate supplies of azithromycin to the WHO campaign, thus relieving some of the campaign's financial burdens.23
 |
| Figure 14. Photograph by Elizabeth Gilbert. |
The first two components of the SAFE strategy for elimination of trachoma utilize simple curative measures that treat active forms of the disease. Surgery, the first of these interventions, targets late stages of the disease and is essential to avoid the latent burden of blindness among adults who have been exposed to trachoma throughout their lifetime.23 Surgery involves a bilamellar tarsal rotation procedure (BTRP) that corrects the eyelid deformities that cause trichiasis. By adjusting the eyelid margin so that the eyelashes are kept from rubbing against the corneal surface, further corneal trauma is prevented and the blinding sequelae is thus averted or at least delayed.1 BTRP is a relatively simple, safe, and quick procedure, and has been performed by nurses with results equal to that of ophthalmologists.1,23
Antibiotics are the second arm of the SAFE strategy, and they specifically target the active, infectious stages of trachoma. It has been shown that a single oral dose of azithromycin can eliminate ocular and extra-ocular infections of C. trachomatis in an individual, thereby halting the cycle of repeated, chronic infections and steadily worsening subtarsal conjunctival scarring.1 The relative simplicity of azithromycin treatment, being a one-time oral medication, ensures a high level of compliance in the field. Furthermore, antibiotic treatment removes possible human reservoirs of C. trachomatis , thus preventing future re-infection on a larger, population-based scale.
The last two components of the SAFE strategy call for facial cleanliness and environmental change. Interventions falling under these two broad categories are essential for a sustained, long-term reduction in trachoma prevalence. This is especially apparent when one looks at the history of trachoma in Europe and North America, where the disease was effectively eliminated by the mid-twentieth century before the advent of powerful antibiotics such as azithromycin and without widespread trichiasis surgery campaigns.1,2 Control of trachoma in these parts of the world came about primarily through a steady increase in the standard of living which was accompanied by improvements in environmental sanitation and behavioral changes with regards to personal hygiene. Such improvements should therefore be considered central to the current effort to eliminate trachoma worldwide.
For the facial cleanliness component of the SAFE strategy, the necessary behavioral changes can be approached in numerous ways, ranging from educating towns and villages about the importance of personal sanitation, to improving access to water, to a mixture of these two interventions. By encouraging facial cleanliness, it is argued that the reservoir of C. trachomatis contained in potentially infectious ocular secretions and nasal exudates is eliminated. This in turn prevents the spread of disease by direct and indirect contact, or by flies that feed on the secretions. A similar argument can be made for other hygienic practices, such as laundry or washing house surfaces in order to prevent indirect transmission of trachoma via fomites.11
 |
|
The final component of the SAFE strategy, environmental change, relates most directly to controlling the population of muscid flies, which serve as vectors for trachoma. One possible intervention involves the use of insecticides to kill the flies, and research has shown that insecticide spraying is an effective means of decreasing muscid fly populations, which in turn leads to a decreased prevalence of trachoma.10 Emerson reported that deltamethrin spraying was costly both financially and in terms of work-hours, therefore making this specific intervention impractical for certain countries or regions.11
Other methods of environmental change that might provide more sustainable means for managing fly vectors include building pit latrines and moving domestic animals to stables outside of the village. Since human feces is the primary choice of breeding media for M sorbens, pit latrines effectively removes their favorable breeding conditions and thereby controls their numbers. This is because M sorbens exhibits a preference for fresh human feces found on the ground, and will not breed in the liquefied excrement found in pit latrines.11,12 Simply building latrines is not sufficient, however, for a behavioral change is also necessary to ensure the use of the latrines. Observations in Mali, for example, note that despite wide adult usage of pit latrines, children continue to deposit feces in the bush not far from the villages, thus allowing the supply of muscid fly larval media to persist.12 The presence of cattle and other animals has also been associated with increased fly density, perhaps through a similar mechanism involving fecal larval media. Removing these animals to holding areas far removed from the settlements is an additional measure to help reduce the population of fly vectors in and around the villages.
6.2 |
The example of Marakissa, The Gambia |
|
|
The strength of environmental change and improved hygiene behavior in decreasing the prevalence of trachoma is perhaps best illustrated by the village of Marakissa in The Gambia.19 Over the 37-year period between trachoma surveys conducted in 1959 and 1996, the prevalence of active inflammatory trachoma dropped from over 65% to 2.4% among children under ten years of age. Similar falls in prevalence were reported for adolescents and adults, with adults showing a zero trachoma prevalence in 1996 as compared to 36.7% in 1959. This vast improvement in eye health, however, occurred in the absence of any interventions aimed at controlling trachoma, such as mass antibiotic campaigns or targeted trichiasis surgery. Rather, the general development and growth of Marakissa resulted in a number of changes that might explain the fall in trachoma prevalence. These changes include improved infrastructure, education and the foundation of a primary school, better access to water, more hygienic washing and bathing habits, the installation and use of pit latrines, and the movement of cattle to areas located outside of the village. Marakissa thus shows that the examples of Europe and North America are repeatable and that changes in hygienic behavior and the environment can be pivotal in upsetting the cycle of endemic trachoma infection.
 |
| Figure 17. Photograph by Elizabeth Gilbert. |
-
Mecaskey JW, Knirsch CA, Kumaresan JA, Cook JA. The possibility of eliminating blinding trachoma. The Lancet Infectious Diseases 2003; 3: 728-34.
-
Mabey DCW, Solomon AW, Foster A. Trachoma. Lancet 2003; 362: 223-29.
-
Darougar S, Forsey T, Jones BR, Allami J, Houshmand A. Isolation of Chlamydia trachomatis from eye secretion (tears). British Journal of Ophthalmology 1979; 63: 256-58.
-
Graczyk TK, Knight R, Gilman RH, Cranfield MR. The role of non-biting flies in the epidemiology of human infectious diseases. Microbes and Infection 2001; 3: 231-35.
-
Solomon AW, Holland MJ, Burton MJ, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet 2003; 362: 198-204.
-
Congdon N, West S, Vitale S, Katala S, Mmbaga BB. Exposure to children and risk of active trachoma in Tanzanian women. American Journal of Epidemiology 1993; 137: 366-72.
-
Burton MJ, Holland MJ, Faal N, et al . Which Members of a Community Need Antibiotics to Control Trachoma? Conjunctival Chlamydia trachomatis Infection Load in Gambian Villages. Investigative Ophthalmology & Visual Science 2003; 44: 4215-22.
-
Forsey T, Darougar S, Transmission of chlamydia by the house fly. British Journal of Ophthalmology 1981; 65: 147-50.
-
Brechner RJ, West S, Lynch M. Trachoma and flies. Individual vs. environmental risk factors. Archives of Ophthalmology 1992; 110: 687-89.
-
Emerson PM, Lindsay SW, Walraven GEL, et al . Effect of fly control on trachoma and diarrhoea. Lancet 1999; 353: 1401-03.
-
Emerson PM, Cairncross S, Bailey RL, Mabey DCW. Review of the evidence base for the 'F' and 'E' components of the SAFE strategy for trachoma control. Tropical Medicine and International Health 2000; 5: 515-27.
-
Schémann JF, Sacko D, Malvy D, et al . Risk factors for trachoma in Mali . International Journal of Epidemiology 2002; 31: 194-201.
-
Sallam TA, Raja'a YA, Al-Zubiery TK, et al . Chlamydia trachomatis infections among Yemeni school pupils in relation to environmental conditions. Saudi Medical Journal 2003; 24: 84-87.
-
Schémann JF, Guinot C, Ilboudo L, et al . Trachoma, flies and environmental factors in Burkina Faso . Transactions of the Royal Society of Tropical Medicine and Hygiene 2003; 97: 63-68.
-
West SK , Congdon N, Katala S, Mele L. Facial cleanliness and risk of trachoma in families. Archives of Ophthalmology 1991; 109: 855-57.
-
West S, Muñoz B, Lynch M, et al . Impact of face-washing on trachoma in Kongwa , Tanzania . Lancet 1995; 345: 155-58.
-
Alene GD, Abebe S. Prevalence of risk factors for trachoma in a rural locality of north-western Ethiopia . East African Medical Journal 2000; 77: 308-12.
-
Emerson PM, Bailey RL, Walraven GEL, Lindsay SW. Human and other faeces as breeding media of the trachoma vector Musca sorbens . Medical and Veterinary Entomology 2001; 15: 314-320.
-
Dolin J, Faal H, Johnson GJ, et al. Reduction of trachoma in a sub-Saharan village in absence of a disease control programme. Lancet 1997; 349: 1511-12.
-
Thylefors B, Negrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bulliten of the World Health Organization 1995; 73: 115-21.
-
Belland RJ, Zhong G, Crane DD, et al . Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis . Proceedings of the National Academy of Sciences 2003; 100: 8478-83.
-
Gérard HC, Freise J, Wang Z, et al. Chlamydia trachomatis genes whose products are related to energy metabolism are expressed differentially in active vs. persistent infection. Microbes and Infection 2002; 4: 13-22.
-
Mabey D, Bailey R. Eradication of trachoma worldwide. British Journal of Ophthalmology 1999; 83: 1261-63.
-
Da Cruz L, Dadour IR, McAllister IL, Jackson A, Isaacs T. Seasonal variation in trachoma and bush flies in north-western Australian Aboriginal communities. Clinical and Experimental Ophthalmology 2002; 30: 80-83.
-
Bailey R, Duong T, Carpenter B, Whittle H, Mabey D. The duration of human ocular chlamydial infection is age-dependent. Epidemiology and Infection 1999; 123: 479-86.
-
West SK , Rapoza P, Munoz B, Katala S, Taylor HR. Epidemiology of ocular chlamydial infection in a trachoma-hyperendemic area. Journal of Infectious Diseases 1991; 163: 752-56.
[Back To Top]
|
|